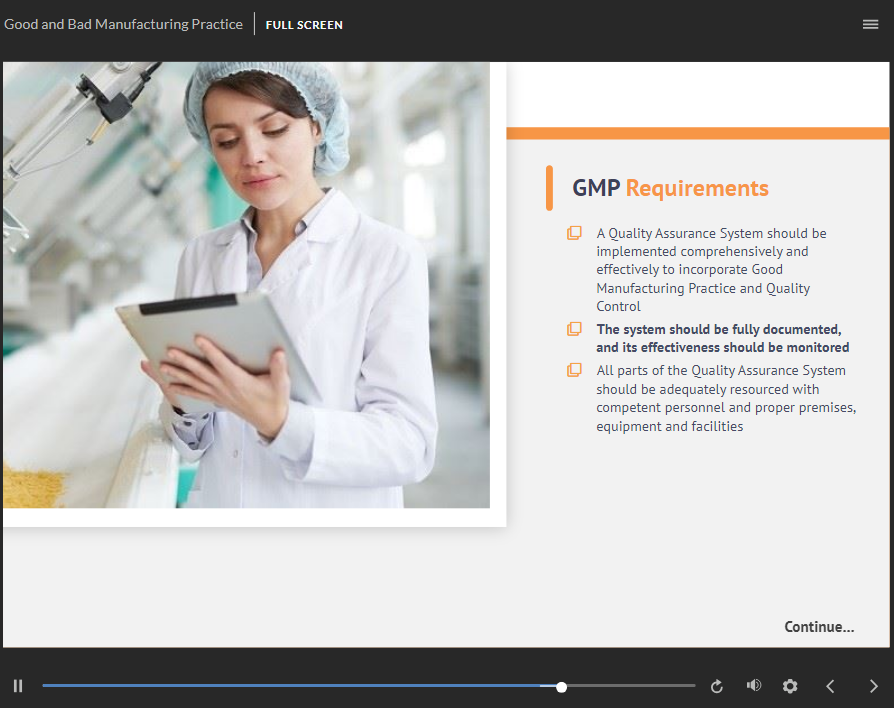
Good Manufacturing Practice Online Course
Good Manufacturing Practice Online Course - Accredited good manufacturing practices trainings designed for life sciences. The digital transformation of manufacturing needs a workforce highly skilled in digital technologies to meet the growing demands of modern manufacturing. Elevate your leadership, amplify your communication skills 1 may,. It covers key principles of the who and pic/s standards and will equip participants with a. Learn the core principles of good manufacturing practices and their importance in ensuring the safety and quality of pharmaceutical products. Let's explore how to design and deliver online employee safety training in manufacturing. Good manufacturing practice course online: Global leadership, lean principles for. The 10 golden rules of gmp is an online training course that overviews regulatory compliance requirements for pharmaceutical production. Whether it's safety essentials or advanced skills. This is 2 day (16 hours) course offered in our training center in chicagoland. Fill in the form below 👇🏼. A comprehensive guide in today’s rapidly evolving pharmaceutical landscape, maintaining compliance with good manufacturing. The digital transformation of manufacturing needs a workforce highly skilled in digital technologies to meet the growing demands of modern manufacturing. Pharmaceutical manufacturers must comply with the regulatory requirements of the countries where they produce and sell medical products. The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good distribution practice (gdp), as. Good manufacturing practice course online: Equip workers with expertly curated manufacturing training from industry leaders. Contact us · managers · manufacturing Reduce your risk of recalls from foreign material and protect your brand. This is 2 day (16 hours) course offered in our training center in chicagoland. Guide to current good manufacturing practices (cgmp) 1 may, 2025; Let's explore how to design and deliver online employee safety training in manufacturing. It covers key principles of the who and pic/s standards and will equip participants with a. A comprehensive guide in today’s rapidly evolving. Fill in the form below 👇🏼. This is 2 day (16 hours) course offered in our training center in chicagoland. It covers key principles of the who and pic/s standards and will equip participants with a. Get an individual certification for your gmp skills. Good manufacturing practice course online: Let's explore how to design and deliver online employee safety training in manufacturing. It requires strategic planning, engaging content, and powerful training software. Fill in the form below 👇🏼. Global leadership, lean principles for. Learn the core principles of good manufacturing practices and their importance in ensuring the safety and quality of pharmaceutical products. Stay ahead of regulatory requirements. A comprehensive guide in today’s rapidly evolving pharmaceutical landscape, maintaining compliance with good manufacturing. The digital transformation of manufacturing needs a workforce highly skilled in digital technologies to meet the growing demands of modern manufacturing. Equip workers with expertly curated manufacturing training from industry leaders. This is 2 day (16 hours) course offered in our. Reduce your risk of recalls from foreign material and protect your brand. Stay ahead of regulatory requirements. It requires strategic planning, engaging content, and powerful training software. Affordable, online training to equip frontline workers. Whether it's safety essentials or advanced skills. Contact us · managers · manufacturing Our course is accredited by the international haccp alliance and recognized by fda, usda and gfsi. Guide to current good manufacturing practices (cgmp) 1 may, 2025; Affordable, online training to equip frontline workers. This course is an introduction to the principles and practices of good manufacturing practices (gmp). Pharmaceutical manufacturers must comply with the regulatory requirements of the countries where they produce and sell medical products. Learn the core principles of good manufacturing practices and their importance in ensuring the safety and quality of pharmaceutical products. Contact us · managers · manufacturing Our course is accredited by the international haccp alliance and recognized by fda, usda and gfsi.. Elevate your leadership, amplify your communication skills 1 may,. Fill in the form below 👇🏼. Good manufacturing practice course online: Get an individual certification for your gmp skills. Our course is accredited by the international haccp alliance and recognized by fda, usda and gfsi. Good manufacturing practice course online: Let's explore how to design and deliver online employee safety training in manufacturing. This free online course provides an overview of essential gmp principles and requirements. Equip workers with expertly curated manufacturing training from industry leaders. A comprehensive guide in today’s rapidly evolving pharmaceutical landscape, maintaining compliance with good manufacturing. Accredited good manufacturing practices trainings designed for life sciences. This is 2 day (16 hours) course offered in our training center in chicagoland. Stay ahead of regulatory requirements. It requires strategic planning, engaging content, and powerful training software. Ensure the safety of your food products with good. Welcome to the certified pharmaceutical gmp professional course, meticulously crafted by cdg to equip professionals with the essential knowledge and skills required to excel in the. This course is an introduction to the principles and practices of good manufacturing practices (gmp). Our course is accredited by the international haccp alliance and recognized by fda, usda and gfsi. Equip workers with expertly curated manufacturing training from industry leaders. A comprehensive guide in today’s rapidly evolving pharmaceutical landscape, maintaining compliance with good manufacturing. Reduce your risk of recalls from foreign material and protect your brand. The european medicines agency's (ema) provides answers to frequently asked questions on good manufacturing practice (gmp) and good distribution practice (gdp), as. Fill in the form below 👇🏼. Good manufacturing practice course online: Elevate your leadership, amplify your communication skills 1 may,. Stay ahead of regulatory requirements. This is 2 day (16 hours) course offered in our training center in chicagoland. Affordable, online training to equip frontline workers. It covers key principles of the who and pic/s standards and will equip participants with a. Pharmaceutical manufacturers must comply with the regulatory requirements of the countries where they produce and sell medical products. Get an individual certification for your gmp skills.5 Key Elements of Good Manufacturing Practices LearnGxP Accredited
Good Manufacturing Practice (GMP) Alpha Academy
Basic Good Manufacturing Practices Guidelines GMP Certification Online
GMP Training Online Course
Introducing Our New Good Manufacturing Practice (GMP) Online Course
Good Manufacturing Practice Training John Academy
Good Manufacturing Practice 01 GMP Online Training Part 1
Good Manufacturing Practice (GMP) in EU Online Certified Course
Good Manufacturing Practices Course Institute for SmallScale Industries
Good Manufacturing Practices (GMP) Online Course (Recorded)
Contact Us · Managers · Manufacturing
The Digital Transformation Of Manufacturing Needs A Workforce Highly Skilled In Digital Technologies To Meet The Growing Demands Of Modern Manufacturing.
Learn The Core Principles Of Good Manufacturing Practices And Their Importance In Ensuring The Safety And Quality Of Pharmaceutical Products.
This Free Online Course Provides An Overview Of Essential Gmp Principles And Requirements.
Related Post: